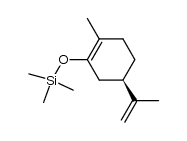
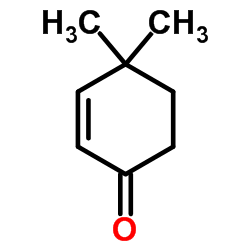
|
~66% |
|
~% |
|
~% |
|
~39% |
|
~50% |
|
~36% |
|
~46% |
|
~47% |
|
~45% |
|
~% |
|
~% |
|
~% |
|
~70% |
|
~71% |
|
~79% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~19% |
|
~% |
|
~% |
|
~% |
|
~73% |
|
~29% |

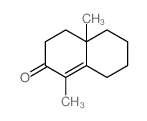


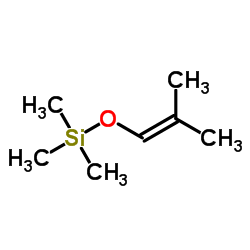

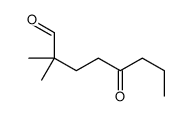

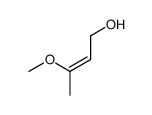
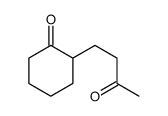
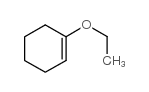
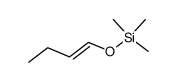
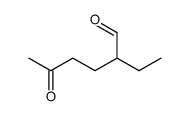
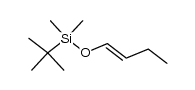
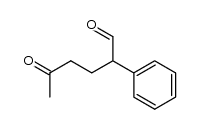
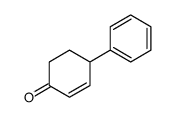
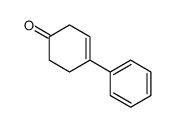
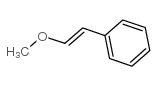
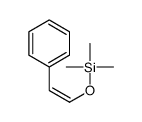
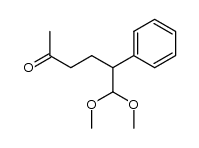
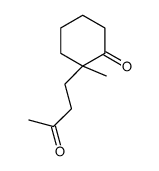
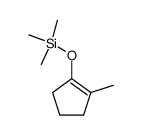
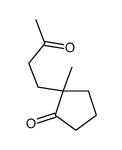
![[1S,4R]-1-methyl-1-(3-pentanone)-4-isopropenyl-2-oxocyclohexane Structure](https://image.chemsrc.com/caspic/074/108032-82-2.png)
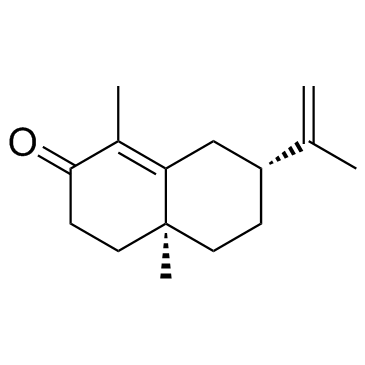
![[4aR,(-)]-4,4a,5,6,7,8-Hexahydro-1,4aα-dimethyl-7β-(1-methylethenyl)naphthalene-2(3H)-one Structure](https://image.chemsrc.com/caspic/185/2303-31-3.png)