| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodium dodecyl sulfate
CAS:151-21-3 |
|
 |
Fentanyl citrate
CAS:990-73-8 |
|
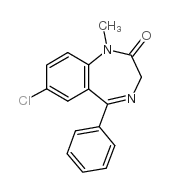 |
diazepam
CAS:439-14-5 |