|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~0% |
|
~% |
|
~% |
|
~% |
|
~53% |
|
~% |
|
~% |
|
~% |
|
~% |

![(2R)-2-{[(E)-(4-methoxyphenyl)methylidene]amino}-2-phenylacetamide Structure](https://image.chemsrc.com/caspic/484/741681-44-7.png)
![(2R)-2-{[(1R)-1-(1,1'-biphenyl)-4-yl-3-butenyl]amino}-2-phenylethanamide Structure](https://image.chemsrc.com/caspic/433/698378-27-7.png)
![(2R)-2-{[(E)-(1,1'-biphenyl)-4-ylmethylidene]amino}-2-phenylethanamide Structure](https://image.chemsrc.com/caspic/234/698377-55-8.png)
![(2R)-2-{[(1R)-1-(2-nitrophenyl)-3-butenyl]amino}-2-phenylethanamide Structure](https://image.chemsrc.com/caspic/492/698378-30-2.png)
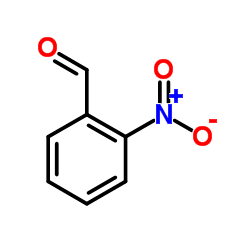

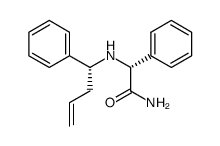
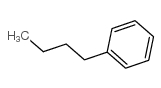
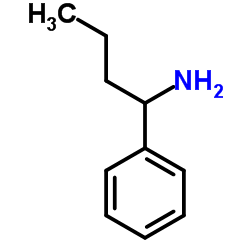
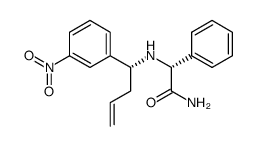

![(2R)-2-{[(E)-(3-nitrophenyl)methylidene]amino}-2-phenylacetamide Structure](https://image.chemsrc.com/caspic/103/698377-63-8.png)
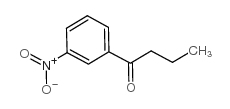
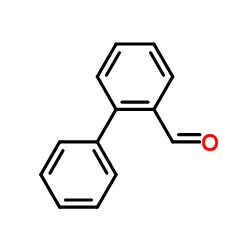

![(2R)-2-{[(E)-(1,1'-biphenyl)-2-ylmethylidene]amino}-2-phenylethanamide Structure](https://image.chemsrc.com/caspic/365/698377-47-8.png)
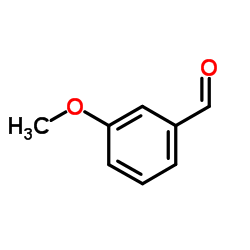
![(2R)-2-{[(E)-(3-methoxyphenyl)methylidene]amino}-2-phenylacetamide Structure](https://image.chemsrc.com/caspic/098/741681-41-4.png)