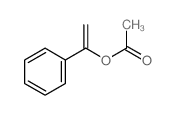
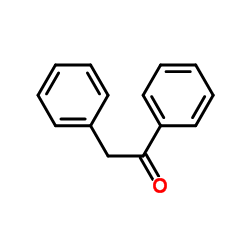
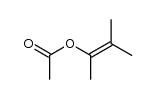
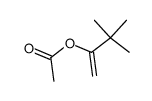
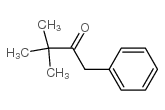
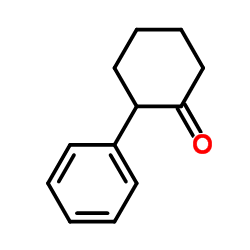
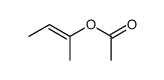
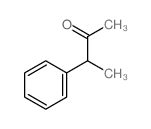
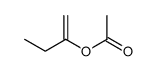
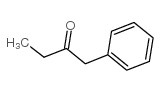
|
~88% |
|
~38% |
|
~% |
|
~84% |
|
~55% |
|
~85% |
|
~61% |
|
~67% |