| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Methanol-d4
CAS:811-98-3 |
|
 |
Methanol
CAS:67-56-1 |
|
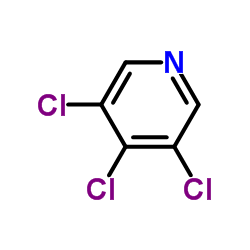 |
3,4,5-Trichloropyridine
CAS:33216-52-3 |
|
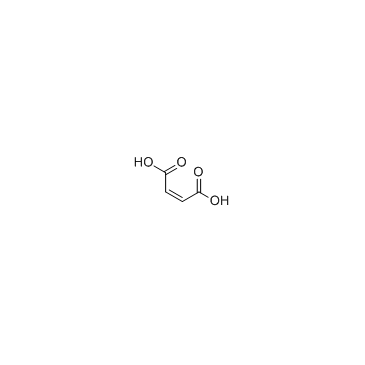 |
Maleic acid
CAS:110-16-7 |