| Structure | Name/CAS No. | Articles |
|---|---|---|
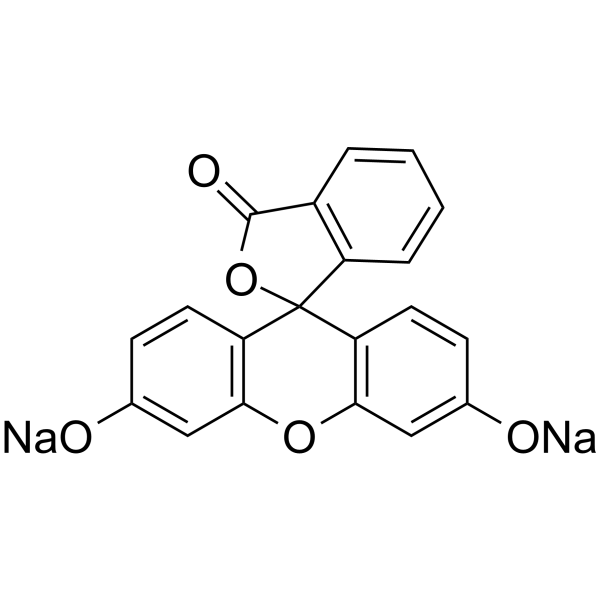 |
Fluorescein sodium
CAS:518-47-8 |
|
 |
Rhodamine 6G
CAS:989-38-8 |
|
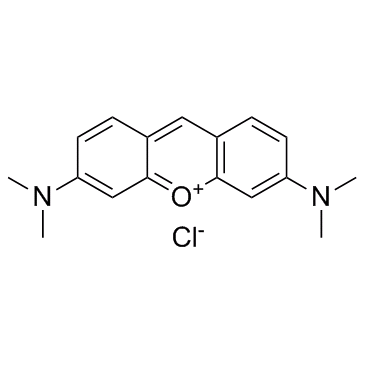 |
Pyronin Y
CAS:92-32-0 |
|
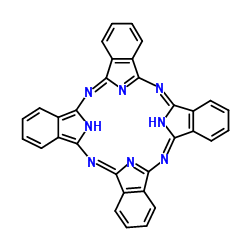 |
PHTHALOCYANINE
CAS:574-93-6 |