| Structure | Name/CAS No. | Articles |
|---|---|---|
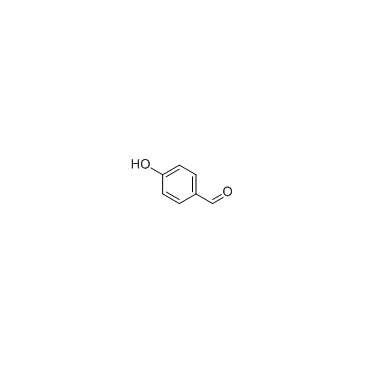 |
4-hydroxybenzaldehyde
CAS:123-08-0 |
|
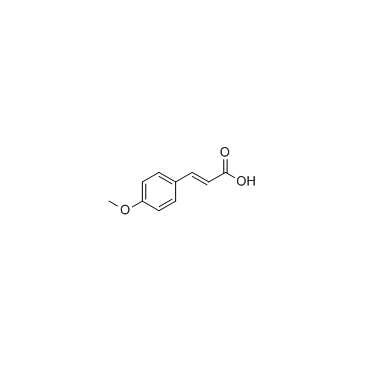 |
4-Methoxycinnamic acid
CAS:830-09-1 |
|
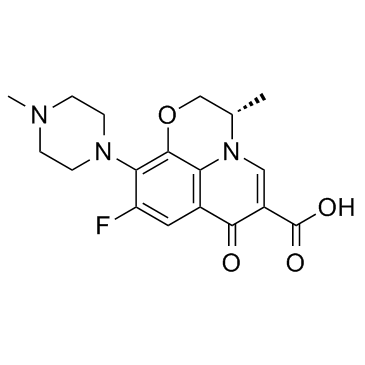 |
Levofloxacin
CAS:100986-85-4 |
|
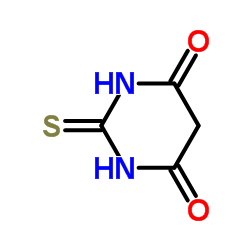 |
4,6-Dihydroxy-2-mercaptopyrimidine
CAS:504-17-6 |