| Structure | Name/CAS No. | Articles |
|---|---|---|
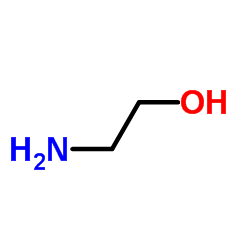 |
2-Aminoethanol
CAS:141-43-5 |
|
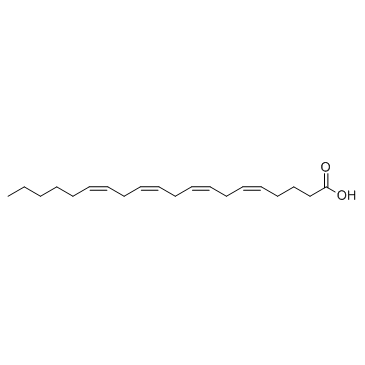 |
Arachidonic acid
CAS:506-32-1 |
|
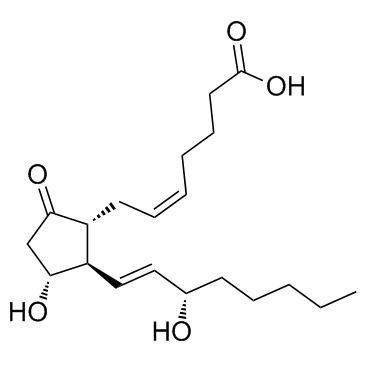 |
Dinoprostone
CAS:363-24-6 |
|
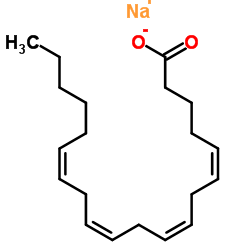 |
Sodium (5Z,8Z,11Z,14Z)-5,8,11,14-icosatetraenoate
CAS:6610-25-9 |