| Structure | Name/CAS No. | Articles |
|---|---|---|
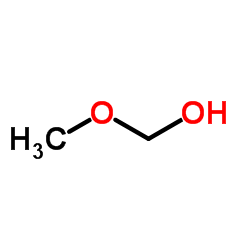 |
Paraformaldehyde
CAS:30525-89-4 |
|
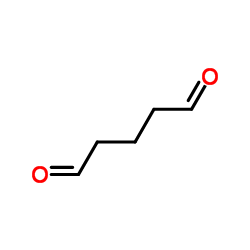 |
glutaraldehyde
CAS:111-30-8 |
|
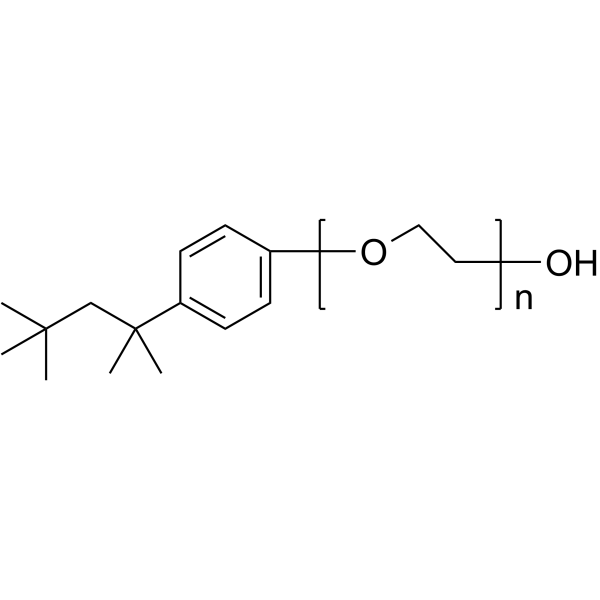 |
Triton X-100
CAS:9002-93-1 |
|
 |
Fibronectin
CAS:86088-83-7 |