| Structure | Name/CAS No. | Articles |
|---|---|---|
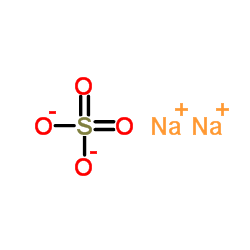 |
sodium sulfate
CAS:7757-82-6 |
|
 |
Sodium sulfate decahydrate
CAS:7727-73-3 |
|
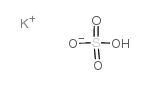 |
Potassium bisulfate
CAS:7646-93-7 |
|
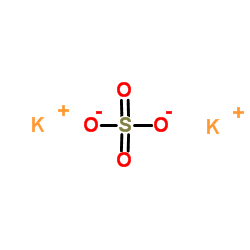 |
Potassium sulfate
CAS:7778-80-5 |
|
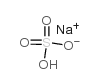 |
Sodium bisulfate
CAS:7681-38-1 |
|
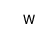 |
Tungsten
CAS:7440-33-7 |
|
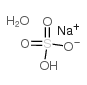 |
Sodium bisulfate monohydrate
CAS:10034-88-5 |