| Structure | Name/CAS No. | Articles |
|---|---|---|
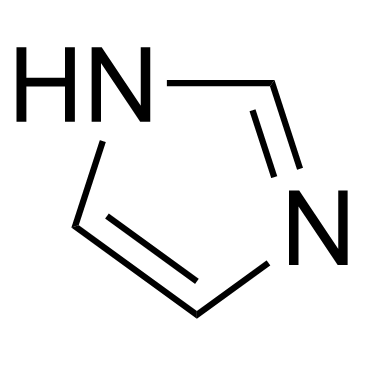 |
Imidazole
CAS:288-32-4 |
|
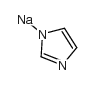 |
Sodium imidazolide
CAS:5587-42-8 |
|
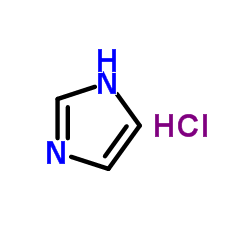 |
1H-Imidazole hydrochloride
CAS:1467-16-9 |