| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Acetonitrile
CAS:75-05-8 |
|
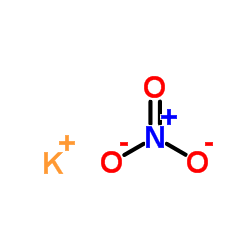 |
Potassium Nitrate
CAS:7757-79-1 |
|
 |
potassium chloride
CAS:7447-40-7 |
|
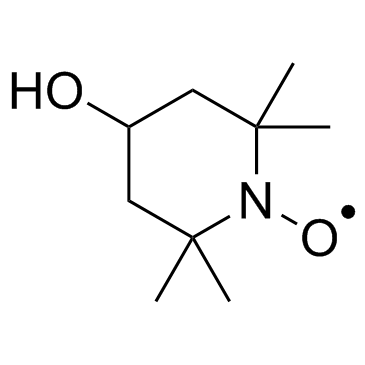 |
Tempol
CAS:2226-96-2 |
|
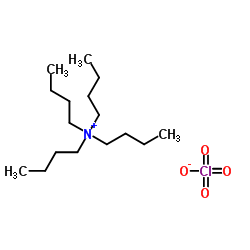 |
Tetrabutylammonium perchlorate
CAS:1923-70-2 |
|
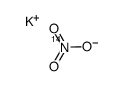 |
potassium,dioxido(oxo)azanium
CAS:1173018-94-4 |
|
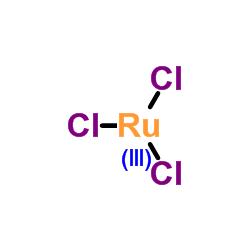 |
ruthenium chloride
CAS:10049-08-8 |