| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
ethyl acetate
CAS:141-78-6 |
|
 |
2-Ethylhexyl nitrate
CAS:27247-96-7 |
|
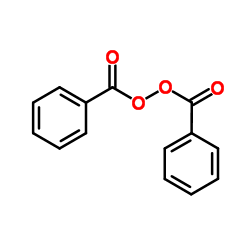 |
Benzoyl peroxide
CAS:94-36-0 |
|
 |
1-Pentadecene
CAS:13360-61-7 |