| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Acetonitrile
CAS:75-05-8 |
|
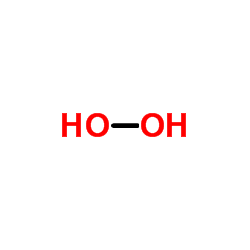 |
Hydrogen peroxide
CAS:7722-84-1 |
|
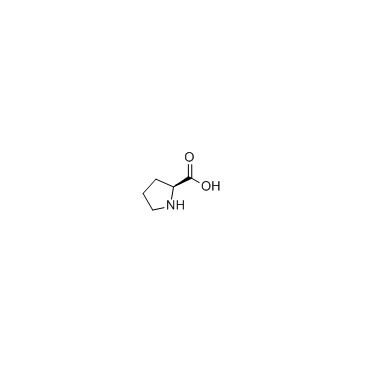 |
Proline
CAS:147-85-3 |
|
 |
Formic Acid
CAS:64-18-6 |
|
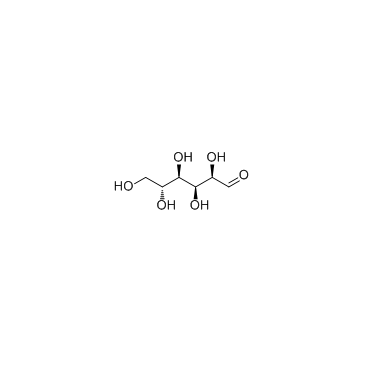 |
D-(+)-Glucose
CAS:50-99-7 |
|
 |
HEPES
CAS:7365-45-9 |
|
 |
Antimony
CAS:7440-36-0 |
|
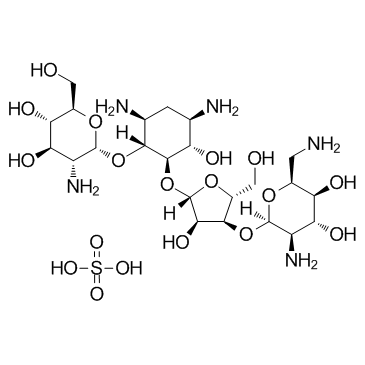 |
Paromomycin Sulfate
CAS:1263-89-4 |
|
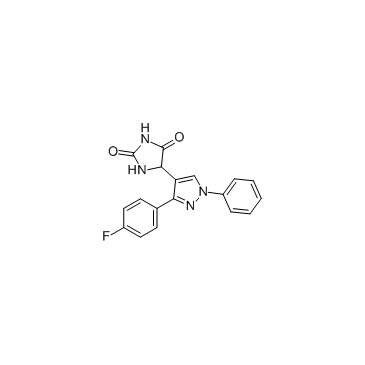 |
DPH
CAS:484049-04-9 |
|
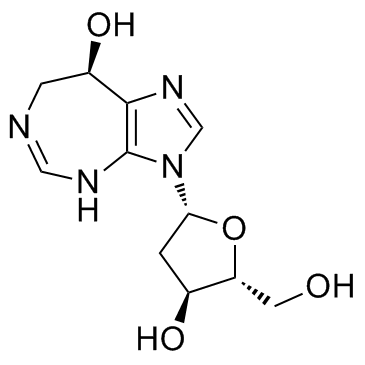 |
PENTOSTATIN
CAS:53910-25-1 |