| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Acetonitrile
CAS:75-05-8 |
|
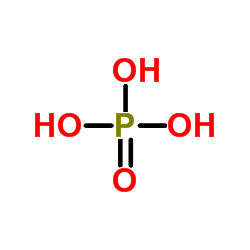 |
Phosphoric acid
CAS:7664-38-2 |
|
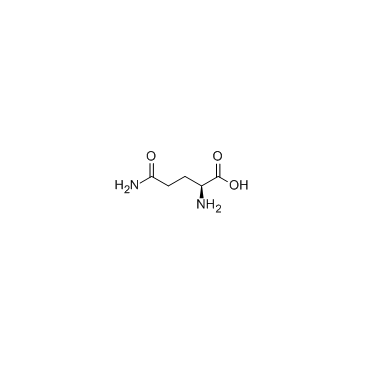 |
L-Glutamine
CAS:56-85-9 |
|
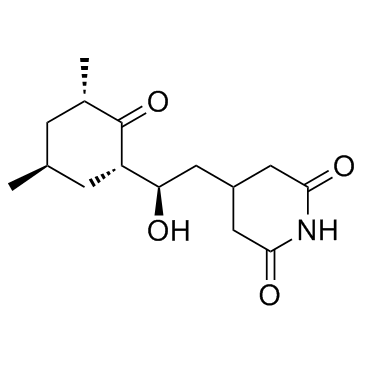 |
Cycloheximide
CAS:66-81-9 |
|
 |
trifluoroacetic acid
CAS:76-05-1 |
|
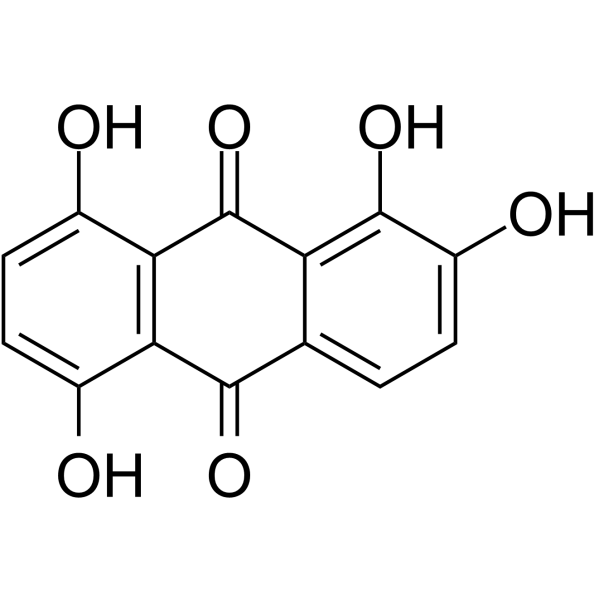 |
Quinalizarin
CAS:81-61-8 |