Kinetic limitations of the Mg(2)Si system for reversible hydrogen storage.
Stephen T Kelly, Sky L Van Atta, John J Vajo, Gregory L Olson, B M Clemens
Index: Nanotechnology 20(20) , 204017, (2009)
Full Text: HTML
Abstract
Despite the promising thermodynamics and storage capacities of many destabilized metal hydride hydrogen storage material systems, they are often kinetically limited from achieving practical and reversible behavior. Such is the case with the Mg2Si system. We investigated the kinetic mechanisms responsible for limiting the reversibility of the MgH2+Si system using thin films as a controlled research platform. We observed that the reaction MgH2 + 1/2Mg2Si + H2 is limited by the mass transport of Mg and Si into separate phases. Hydrogen readily diffuses through the Mg2Si material and nucleating MgH2 phase growth does not result in reaction completion. By depositing and characterizing multilayer films of Mg2Si and Mg with varying Mg2Si layer thicknesses, we conclude that the hydrogenation reaction consumes no more than 1 nm of Mg2Si, making this system impractical for reversible hydrogen storage.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
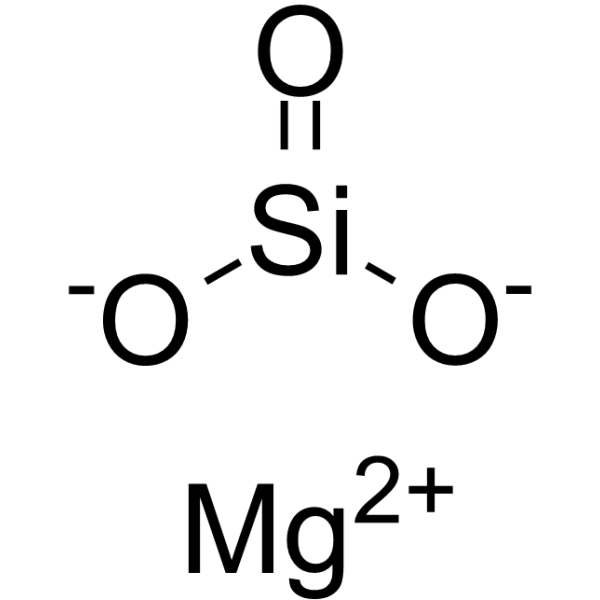 |
Magnesium silicate
CAS:1343-88-0 |
MgO3Si |
|
Effects of powder flow properties on capsule filling weight ...
2013-09-01 [Drug Dev. Ind. Pharm. 39(9) , 1464-75, (2013)] |
|
Supramolecular dynamics of thalidomide and its derivatives i...
2010-05-05 [Chirality 22(4) , 416-24, (2010)] |
|
Single-crystal structure determination of (Mg,Fe)SiO3 postpe...
2013-04-16 [Proc. Natl. Acad. Sci. U. S. A. 110(16) , 6292-5, (2013)] |
|
Occurrence and fate of organochlorinated pesticides and PAH ...
2009-08-01 [Arch. Environ. Contam. Toxicol. 57(2) , 247-55, (2009)] |
|
Covalent immobilization of catalase onto spacer-arm attached...
2011-12-10 [Enzyme Microb. Technol. 49(6-7) , 547-54, (2011)] |