| Structure | Name/CAS No. | Articles |
|---|---|---|
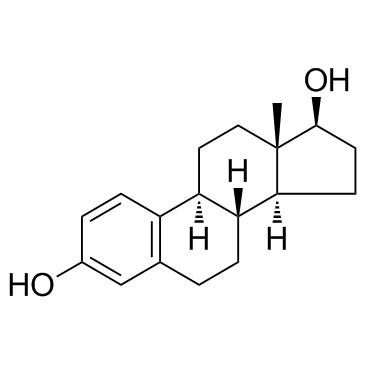 |
beta-Estradiol
CAS:50-28-2 |
|
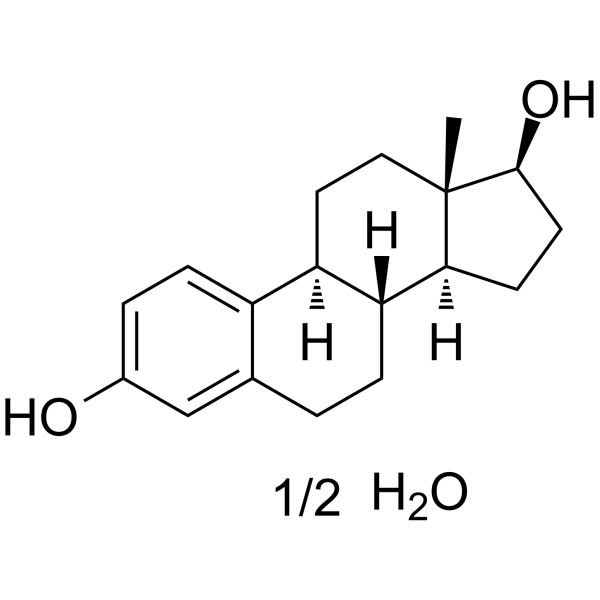 |
ESTRADIOL HEMIHYDRATE
CAS:35380-71-3 |
|
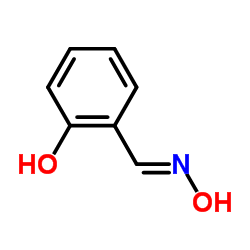 |
salicylaldehyde, oxime
CAS:94-67-7 |