Molecules
2012-01-01
Synthesis of a cholesteryl-HEG phosphoramidite derivative and its application to lipid-conjugates of the anti-HIV 5'TGGGAG³' Hotoda's sequence.
Domenica Musumeci, Daniela Montesarchio
Index: Molecules 17(10) , 12378-92, (2012)
Full Text: HTML
Abstract
A novel phosphoramidite derivative of cholesterol, with an ether-linked hexaethylene glycol (HEG) spacer arm, has been obtained through simple and reproducible solid phase modified oligonucleotide synthesis manipulations. This building block and the known phosphoramidite derivative of 3b-(2-hydroxyethoxy)cholesterol have been exploited in standard oligonucleotide synthesis protocols for the preparation of 5'- conjugates of the G-quadruplex-forming ⁵'TGGGAG³' oligomer, known as the Hotoda's sequence, to produce new potential anti-HIV agents.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
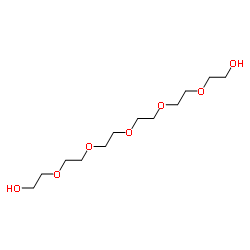 |
hexaethyleneglycol
CAS:2615-15-8 |
C12H26O7 |
Related Articles:
More...
|
Synthesis and evaluation of new spacers for use as dsDNA end...
2010-08-18 [Bioconjug. Chem. 21(8) , 1545-53, (2010)] |
|
Adsorption isotherms of aqueous C12E6 and cetyltrimethylammo...
2009-05-19 [Langmuir 25(10) , 5536-44, (2009)] |
|
A nicked duplex decamer DNA with a PEG(6) tether.
2001-03-01 [Nucleic Acids Res. 29(5) , 1132-43, (2001)] |
|
Design and synthesis of RNA miniduplexes via a synthetic lin...
1993-02-23 [Biochemistry 32(7) , 1751-8, (1993)] |
|
Selectively 13C-enriched DNA: 13C and 1H assignments of a tr...
1994-07-01 [J. Biomol. NMR 4(4) , 575-80, (1994)] |