| Structure | Name/CAS No. | Articles |
|---|---|---|
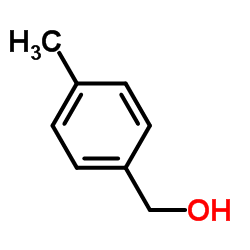 |
p-tolylmethanol
CAS:589-18-4 |
Carla Villa, Beatrice Trucchi, Raffaella Gambaro, Sara Baldassari
Index: Int. J. Cosmet. Sci. 30(2) , 139-44, (2008)
Full Text: HTML
Several alcohols--interesting as cosmetic fragrances whose main preparative route on an industrial scale or in the research laboratory is the reduction of the corresponding carbonyl compound--were obtained by a solvent-free methodology in a green chemistry context. The process involves the simple mixing of the carbonyl compound with sodium borohydride dispersed in wet alumina in the solid state; the conversions of the carbonyl compounds were obtained in good yields within short reaction times, without energy consumption. The following carbinols were studied: octan-3-ol, 2-cineolylols (endo-exo mixture), alpha-ionol, 4-methylbenzyl alcohol, 1-phenylethanol, trans-cinnamyl alcohol, p-anisyl alcohol, 4-phenyl-3-buten-2-ol.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
p-tolylmethanol
CAS:589-18-4 |
C8H10O |
|
Polycarbonates derived from glucose via an organocatalytic a...
2013-05-08 [J. Am. Chem. Soc. 135(18) , 6826-9, (2013)] |
|
Assembly of Mesoporous Metal-Organic Framework Templated by ...
2015-08-03 [ChemPhysChem 16 , 2317-21, (2015)] |
|
Pd/C-catalyzed chemoselective hydrogenation in the presence ...
2003-03-01 [Chem. Pharm. Bull. 51(3) , 320-4, (2003)] |
|
Effect of p-xylene metabolites, p-methylbenzyl alcohol and 2...
1992-04-01 [Res. Commun. Chem. Pathol. Pharmacol. 76(1) , 117-20, (1992)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved