| Structure | Name/CAS No. | Articles |
|---|---|---|
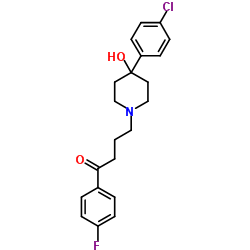 |
Ethoxylated hydrogenated castor oil
CAS:61788-85-0 |
|
 |
Cremophor EL
CAS:61791-12-6 |