| Structure | Name/CAS No. | Articles |
|---|---|---|
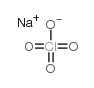 |
Sodium perchlorate
CAS:7601-89-0 |
|
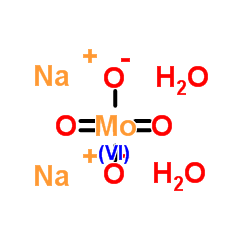 |
sodium molybdate dihydrate
CAS:10102-40-6 |
|
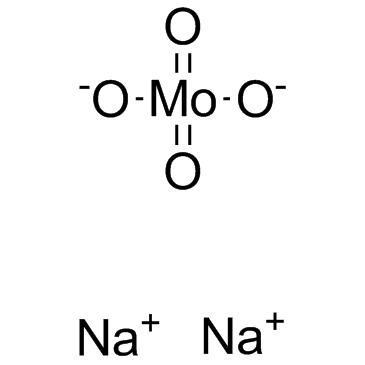 |
Sodium molybdate
CAS:7631-95-0 |