| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Malachite green oxalate
CAS:2437-29-8 |
|
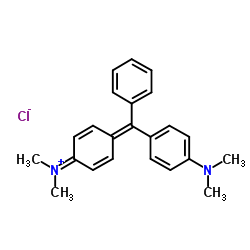 |
Malachite green
CAS:569-64-2 |
|
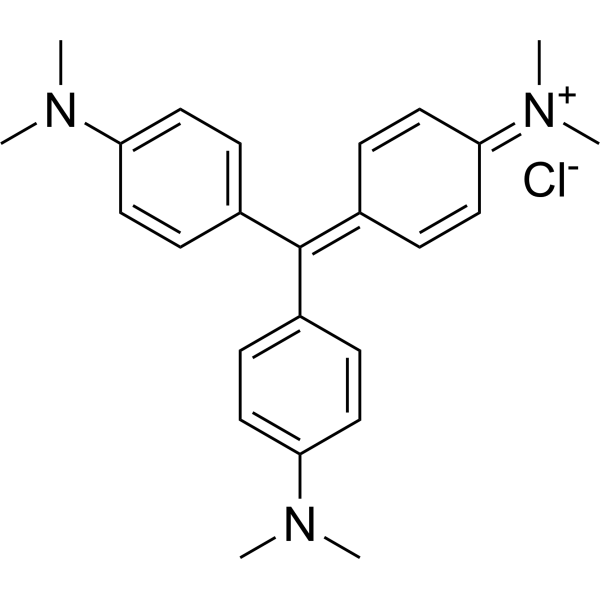 |
Crystal Violet
CAS:548-62-9 |
|
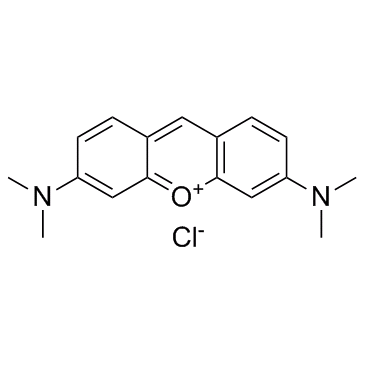 |
Pyronin Y
CAS:92-32-0 |