| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Glycerol
CAS:56-81-5 |
|
 |
Dimethyl sulfoxide
CAS:67-68-5 |
|
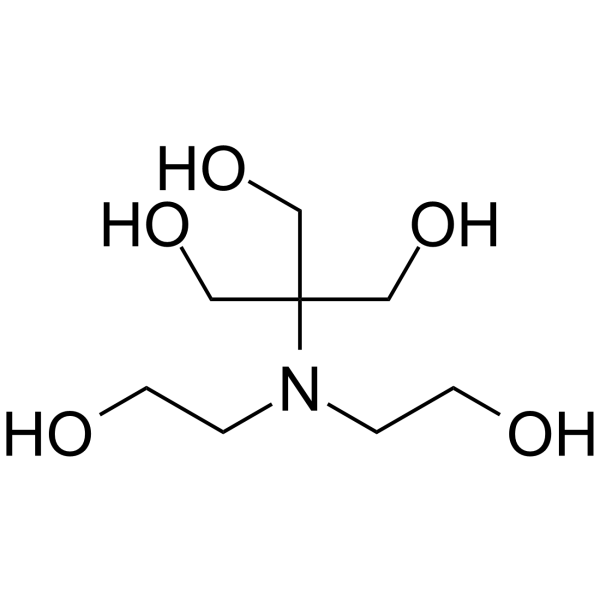 |
Bis-tris methane
CAS:6976-37-0 |
|
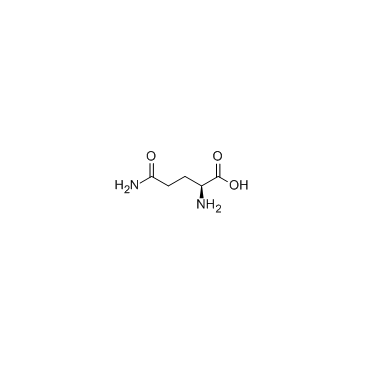 |
L-Glutamine
CAS:56-85-9 |
|
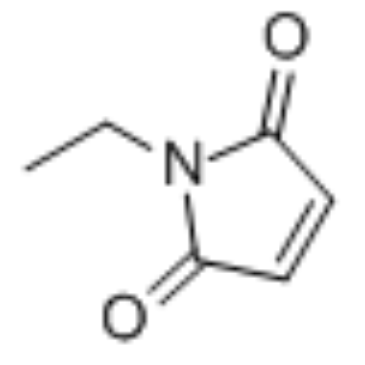 |
N-ethylmaleimide
CAS:128-53-0 |
|
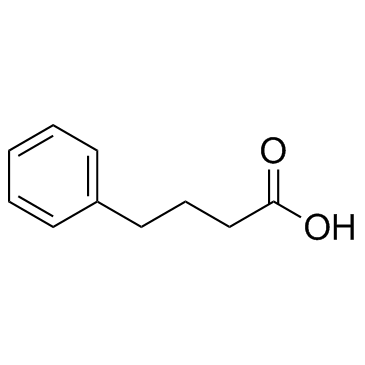 |
4-Phenylbutyric acid
CAS:1821-12-1 |
|
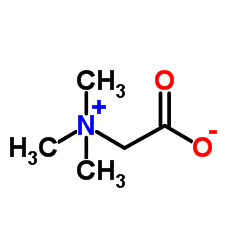 |
Betaine
CAS:107-43-7 |
|
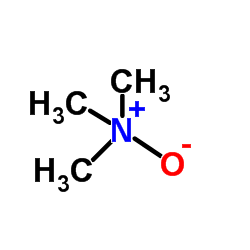 |
Trimethylamine oxide
CAS:1184-78-7 |
|
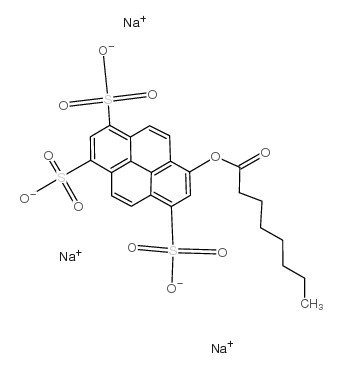 |
8-Octanoyloxypyrene-1,3,6-trisulfonic acid trisodium salt
CAS:115787-84-3 |