| Structure | Name/CAS No. | Articles |
|---|---|---|
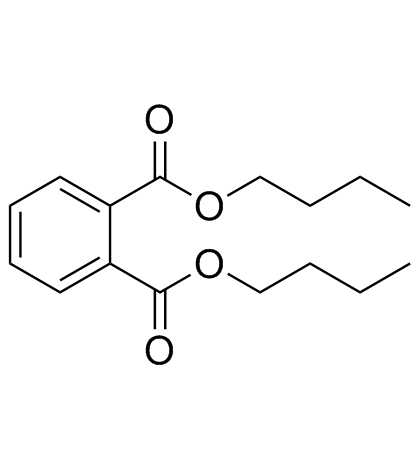 |
1,2-Benzenedicarboxylic acid
CAS:84-74-2 |
|
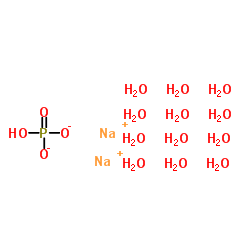 |
Disodium phosphate dodecahydrate
CAS:10039-32-4 |
|
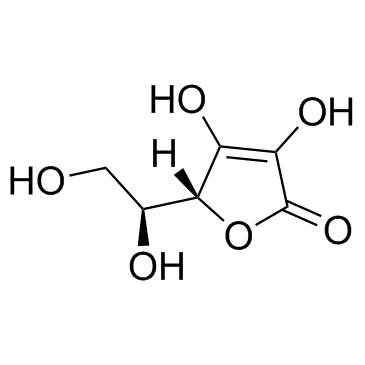 |
Ascorbic acid
CAS:50-81-7 |
|
 |
Aluminum potassium sulfate 12-hydrate
CAS:7784-24-9 |
|
![Bisbenz[5,6]indeno[1,2,3-cd:1',2',3'-lM]perylene, 5,10,15,20-tetraphenyl Structure](https://image.chemsrc.com/caspic/164/175606-05-0.png) |
Bisbenz[5,6]indeno[1,2,3-cd:1',2',3'-lM]perylene, 5,10,15,20-tetraphenyl
CAS:175606-05-0 |
|
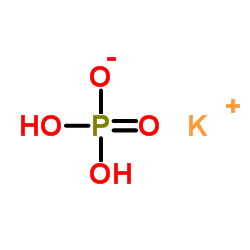 |
Monopotassium phosphate
CAS:7778-77-0 |
|
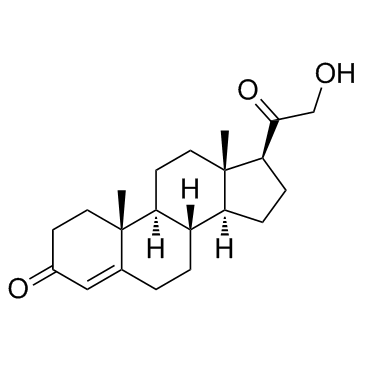 |
Desoxycorticosterone
CAS:64-85-7 |
|
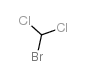 |
BROMODICHLOROMETHANE
CAS:75-27-4 |