| Structure | Name/CAS No. | Articles |
|---|---|---|
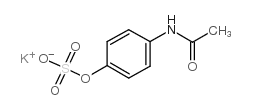 |
paracetamol sulfate potassium salt
CAS:32113-41-0 |
|
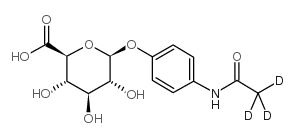 |
acetaminophen glucuronide
CAS:16110-10-4 |