| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Ketoconazole
CAS:65277-42-1 |
|
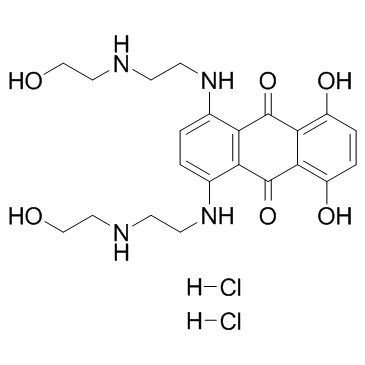 |
Mitoxantrone 2HCl
CAS:70476-82-3 |