| Structure | Name/CAS No. | Articles |
|---|---|---|
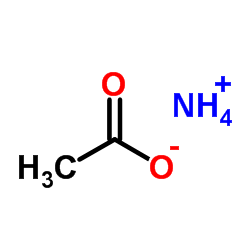 |
Ammonium acetate
CAS:631-61-8 |
|
 |
Methanol
CAS:67-56-1 |
|
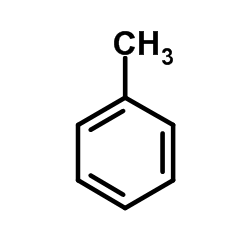 |
Toluene
CAS:108-88-3 |
|
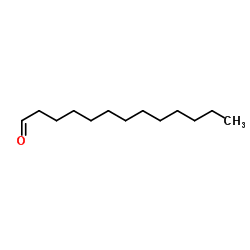 |
Tridecanal
CAS:10486-19-8 |
|
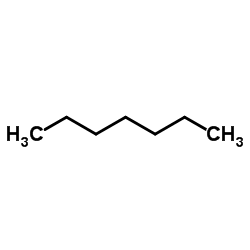 |
Heptane
CAS:142-82-5 |