| Structure | Name/CAS No. | Articles |
|---|---|---|
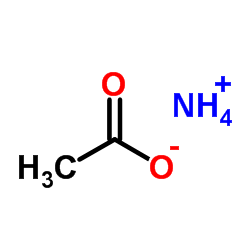 |
Ammonium acetate
CAS:631-61-8 |
|
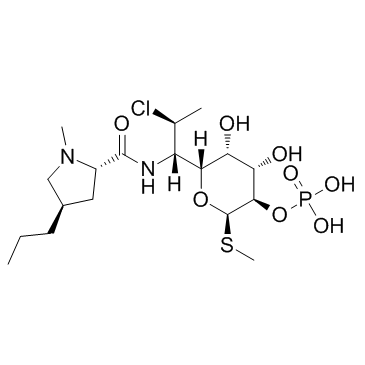 |
Clindamycin phosphate
CAS:24729-96-2 |