| Structure | Name/CAS No. | Articles |
|---|---|---|
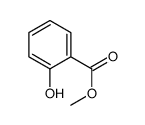 |
Methyl salicylate
CAS:90045-28-6 |
|
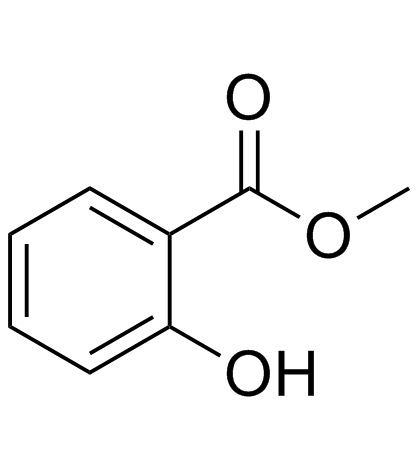 |
Methyl salicylate
CAS:119-36-8 |
|
 |
Aspirin
CAS:50-78-2 |