Organic Letters
2009-04-02
Functional characterization of ttmM unveils new tautomycin analogs and insight into tautomycin biosynthesis and activity.
Jianhua Ju, Wenli Li, Qiuping Yuan, Noel R Peters, F Michael Hoffmann, Scott R Rajski, Hiroyuki Osada, Ben Shen
Index: Org. Lett. 11(7) , 1639-42, (2009)
Full Text: HTML
Abstract
The biosynthetic gene cluster for tautomycin (TTM), a potent protein phosphatase (PP) inhibitor has recently been characterized. Inactivation of ttmM, which encodes a putative C3' hydroxylase, afforded mutant SB6005 which accumulated three new 3'-deshydroxy TTM analogs, supporting the function of TtmM and the previously proposed linear pathway for TTM biosynthesis. Bioassays reveal the importance of the C3' OH moiety in PP inhibition and that PP inhibition is not the exclusive mechanism driving TTM-induced cell death.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
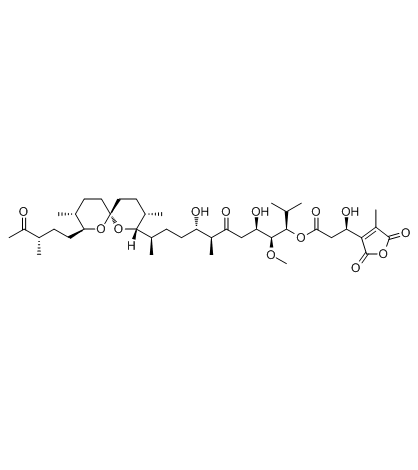 |
Tautomycin
CAS:109946-35-2 |
C41H66O13 |
Related Articles:
More...
|
Characterization of the tautomycetin biosynthetic gene clust...
2009-03-27 [J. Nat. Prod. 72(3) , 450-9, (2009)] |
|
The bifunctional glyceryl transferase/phosphatase OzmB belon...
2006-08-16 [J. Am. Chem. Soc. 128(32) , 10386-7, (2006)] |
|
PR55 alpha, a regulatory subunit of PP2A, specifically regul...
2009-08-21 [J. Biol. Chem. 284 , 22649 - 22656, (2009)] |
|
Tautomycin suppresses growth and neuroendocrine hormone mark...
2009-01-01 [Am. J. Surg. 197(3) , 313-9, (2009)] |
|
Tautomycin's interactions with protein phosphatase 1.
2010-03-01 [Chem. Asian J. 5(3) , 410-20, (2010)] |