| Structure | Name/CAS No. | Articles |
|---|---|---|
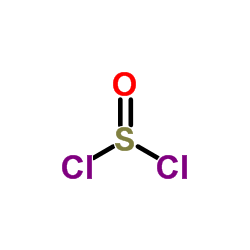 |
Thionyl chloride
CAS:7719-09-7 |
|
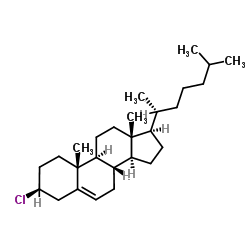 |
CHOLESTEROL CHLORIDE
CAS:910-31-6 |