| Structure | Name/CAS No. | Articles |
|---|---|---|
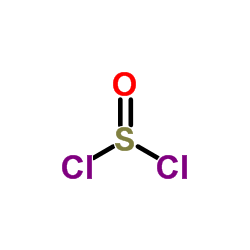 |
Thionyl chloride
CAS:7719-09-7 |
|
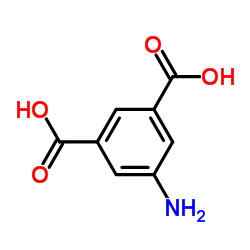 |
5-Aminoisophthalic acid
CAS:99-31-0 |
|
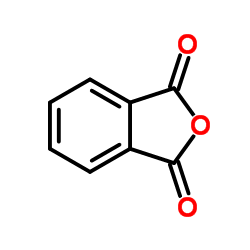 |
Phthalic anhydride
CAS:85-44-9 |