| Structure | Name/CAS No. | Articles |
|---|---|---|
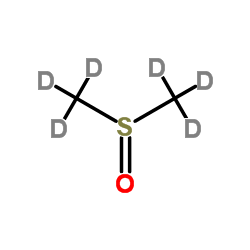 |
DIMETHYL SULFOXIDE-D6
CAS:2206-27-1 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
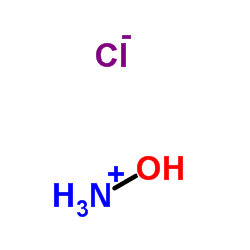 |
Hydroxyamine hydrochloride
CAS:5470-11-1 |
|
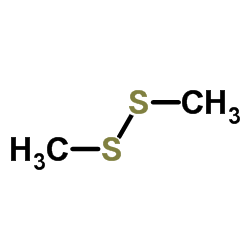 |
Dimethyl disulfide
CAS:624-92-0 |
|
 |
Sodium methanesulfinate
CAS:20277-69-4 |