| Structure | Name/CAS No. | Articles |
|---|---|---|
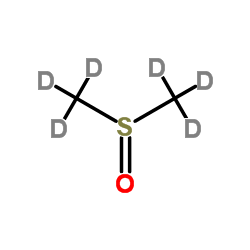 |
DIMETHYL SULFOXIDE-D6
CAS:2206-27-1 |
|
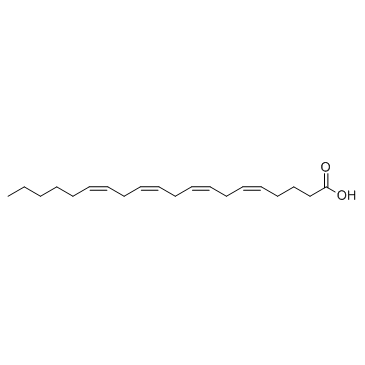 |
Arachidonic acid
CAS:506-32-1 |
|
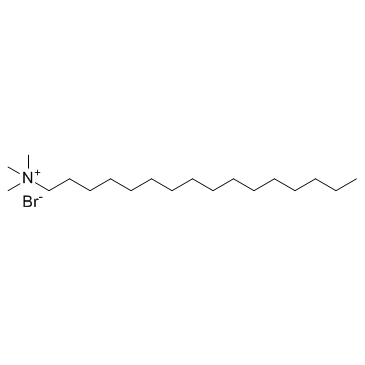 |
Hexadecyl trimethyl ammonium bromide
CAS:57-09-0 |
|
 |
2,3-Dimethylphenol
CAS:526-75-0 |