| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
D-3-phenyllactic acid
CAS:7326-19-4 |
|
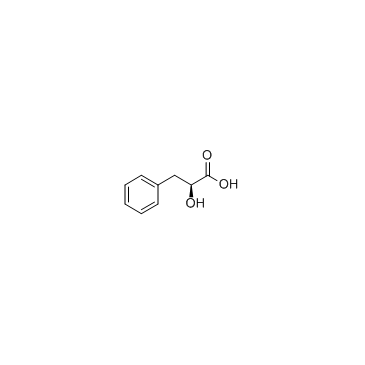 |
L-(-)-3-Phenyllactic acid
CAS:20312-36-1 |
|
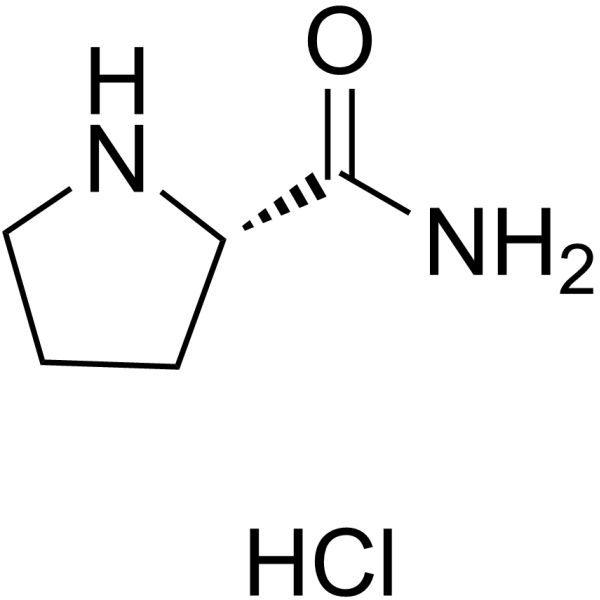 |
H-Pro-NH2.HCl
CAS:42429-27-6 |
|
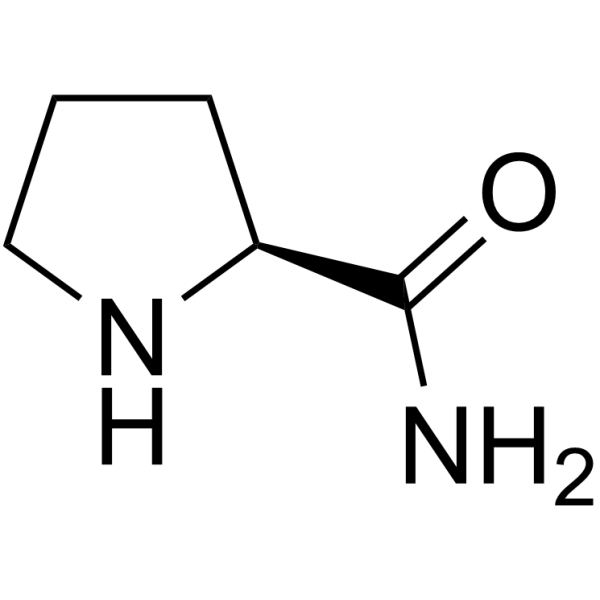 |
L-Prolinamide
CAS:7531-52-4 |
|
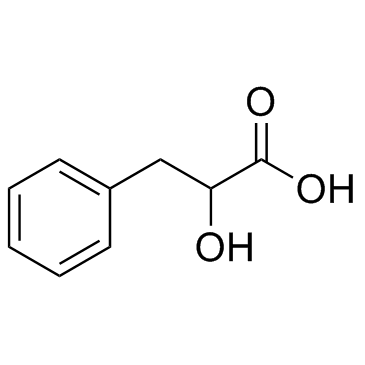 |
D-3-phenyllactic acid
CAS:828-01-3 |