| Structure | Name/CAS No. | Articles |
|---|---|---|
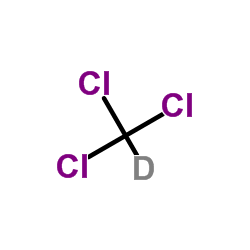 |
chloroform-d
CAS:865-49-6 |
|
 |
Methanol
CAS:67-56-1 |
|
 |
Dichloromethane
CAS:75-09-2 |
|
 |
Dimethyl but-2-ynedioate
CAS:762-42-5 |