| Structure | Name/CAS No. | Articles |
|---|---|---|
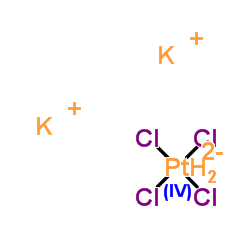 |
Potassium tetrachloroplatinate(II)
CAS:10025-99-7 |
|
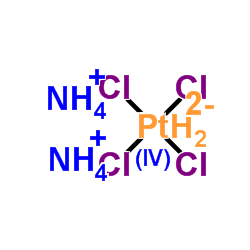 |
Platinum(II)-ammonium chloride
CAS:13820-41-2 |