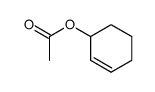
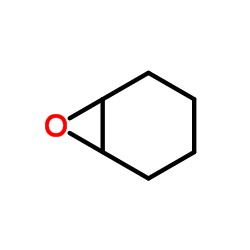
|
~80% |
|
~87% |
|
~81% |
|
~78% |
|
~82% |
|
~58% |
|
~75% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~85% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
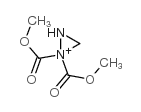
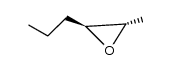
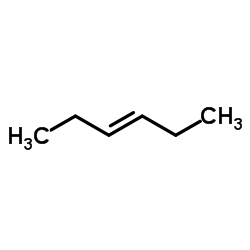

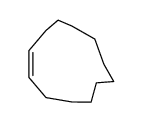
![14-Oxabicyclo[11.1.0]tetradecane Structure](https://image.chemsrc.com/caspic/327/286-99-7.png)

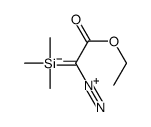
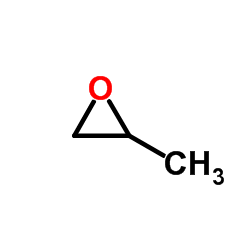
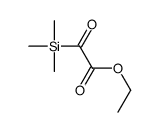
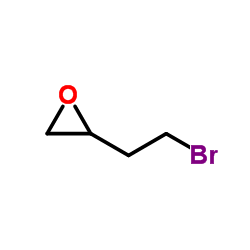
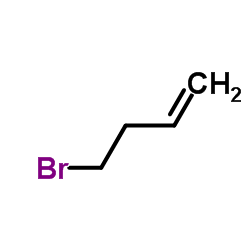
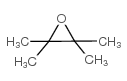
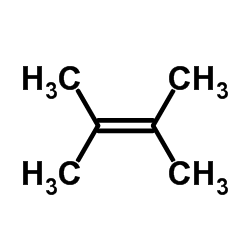
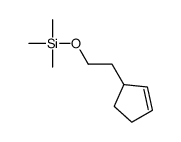
![Silane, trimethyl[2-(6-oxabicyclo[3.1.0]hex-2-yl)ethoxy]- (9CI) Structure](https://image.chemsrc.com/caspic/040/89608-55-9.png)