MMP regulation of corneal keratocyte motility and mechanics in 3-D collagen matrices.
Chengxin Zhou, W Matthew Petroll
Index: Exp. Eye Res. 121 , 147-60, (2014)
Full Text: HTML
Abstract
Previous studies have shown that platelet derived growth factor (PDGF) can stimulate corneal keratocyte spreading and migration within 3-D collagen matrices, without inducing transformation to a contractile, fibroblastic phenotype. The goal of this study was to investigate the role of matrix metalloproteinases (MMPs) in regulating PDGF-induced changes in keratocyte motility and mechanical differentiation. Rabbit corneal keratocytes were isolated and cultured in serum-free media (S-) to maintain their quiescent phenotype. A nested collagen matrix construct was used to assess 3-D cell migration, and a standard collagen matrix model was used to assess cell morphology and cell-mediated matrix contraction. In both cases constructs were cultured in S- supplemented with PDGF, with or without the broad spectrum MMP inhibitors GM6001 or BB-94. After 4 days, f-actin, nuclei and collagen fibrils were imaged using confocal microscopy. To assess sub-cellular mechanical activity (extension and retraction of cell processes), time-lapse DIC imaging was also performed. MT1-MMP expression and MMP-mediated collagen degradation were also examined. Results demonstrated that neither GM6001 nor BB-94 affected corneal keratocyte viability or proliferation in 3-D culture. PDGF stimulated elongation and migration of corneal keratocytes within type I collagen matrices, without causing a loss of their dendritic morphology or inducing formation of intracellular stress fibers. Treatment with GM6001 and BB-94 inhibited PDGF-induced keratocyte spreading and migration. Relatively low levels of keratocyte-induced matrix contraction were also maintained in PDGF, and the amount of PDGF-induced collagen degradation was similar to that observed in S- controls. The collagen degradation pattern was consistent with membrane-associated MMP activity, and keratocytes showed positive staining for MT1-MMP, albeit weak. Both matrix contraction and collagen degradation were reduced by MMP inhibition. For most outcome measures, the inhibitory effect of BB-94 was significantly greater than that of GM6001. Overall, the data demonstrate for the first time that even under conditions in which low levels of contractility and extracellular matrix proteolysis are maintained, MMPs still play an important role in mediating cell spreading and migration within 3-D collagen matrices. This appears to be mediated at least in part by membrane-tethered MMPs, such as MT1-MMP. Copyright © 2014 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
L-Phenylalanine
CAS:63-91-2 |
C9H11NO2 | |
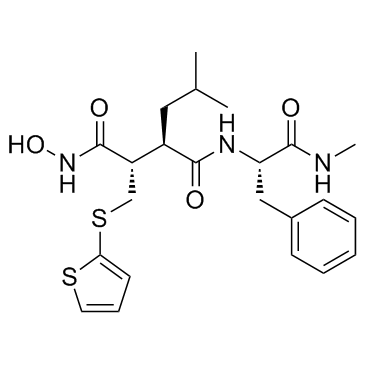 |
Batimastat
CAS:130370-60-4 |
C23H31N3O4S2 |
|
Assessment of protein modifications in liver of rats under c...
2015-04-29 [J. Proteomics 120 , 194-203, (2015)] |
|
Enhanced Indirect Photochemical Transformation of Histidine ...
2015-05-05 [Environ. Sci. Technol. 49 , 5511-9, (2015)] |
|
Use of Commercial Dry Yeast Products Rich in Mannoproteins f...
2015-06-17 [J. Agric. Food Chem. 63 , 5670-81, (2015)] |
|
The human serum metabolome.
2011-01-01 [PLoS ONE 6(2) , e16957, (2011)] |
|
Renal clearance of branched-chain L-amino and 2-oxo acids in...
1999-08-01 [J. Inherit. Metab. Dis. 22(6) , 706-22, (1999)] |