| Structure | Name/CAS No. | Articles |
|---|---|---|
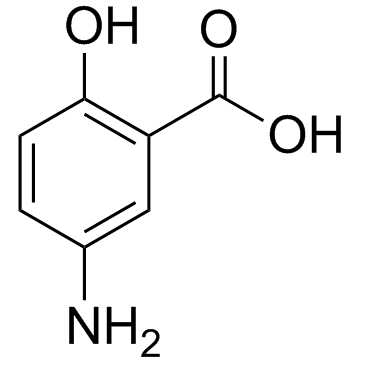 |
5-Aminosalicylic Acid
CAS:89-57-6 |
|
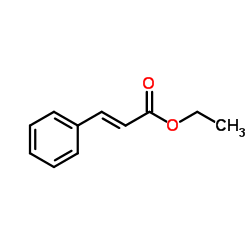 |
Ethyl cinnamate
CAS:103-36-6 |
|
 |
Aspirin
CAS:50-78-2 |
|
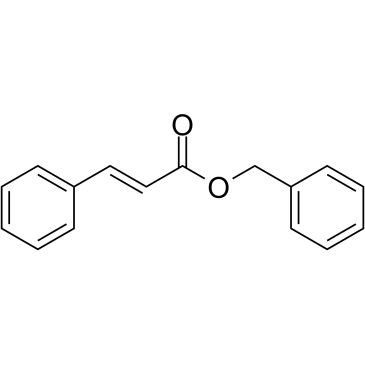 |
Benzyl cinnamate
CAS:103-41-3 |