Divergent approach to the bisanthraquinone natural products: total synthesis of (S)-bisoranjidiol and derivatives from binaphtho-para-quinones.
Erin E Podlesny, Marisa C Kozlowski
Index: J. Org. Chem. 78(2) , 466-76, (2013)
Full Text: HTML
Abstract
The development of the first asymmetric synthesis of a chiral anthraquinone dimer is outlined, resulting in the first total synthesis of (S)-bisoranjidiol. Rather than a biomimetic dimerization retrosynthetic disconnection, the anthracenyl ring systems are generated after formation of the axially chiral binaphthalene framework. This synthetic strategy has enabled the synthesis of several analogues. Key features of the synthesis include the enantioselective coupling of a hindered 2-naphthol containing substitution peri to the site of C-C bond formation, the regioselective oxidation of 8,8'-hydroxylated binaphthols to binaphtho-para-quinones, and a tandem regioselective Diels-Alder/aromatization reaction.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
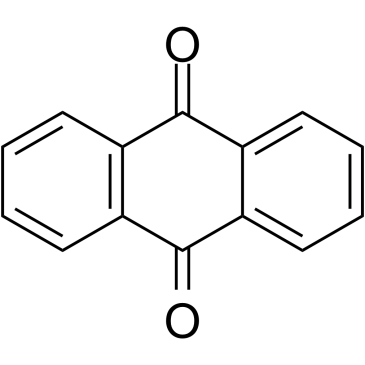 |
Anthraquinone
CAS:84-65-1 |
C14H8O2 |
|
Antimicrobial mechanism of resveratrol-trans-dihydrodimer pr...
2015-12-01 [Biotechnol. Bioeng. 112 , 2417-28, (2015)] |
|
Gas chromatography with parallel hard and soft ionization ma...
2015-01-15 [Rapid Commun. Mass Spectrom. 29(1) , 91-9, (2014)] |
|
Comparative study of alkaline hydrogen peroxide and organoso...
2015-01-01 [Bioresour. Technol. 187 , 161-6, (2015)] |
|
Measuring the relative hydrogen-bonding strengths of alcohol...
2015-01-12 [ChemPhysChem 16(1) , 160-8, (2015)] |
|
A new method and tool for detection and quantification of PM...
2015-08-01 [Environ. Sci. Pollut. Res. Int. 22 , 12469-78, (2015)] |