| Structure | Name/CAS No. | Articles |
|---|---|---|
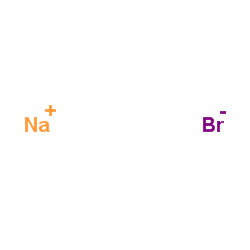 |
Sodium bromide
CAS:7647-15-6 |
|
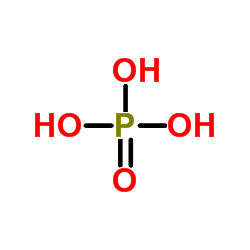 |
Phosphoric acid
CAS:7664-38-2 |
|
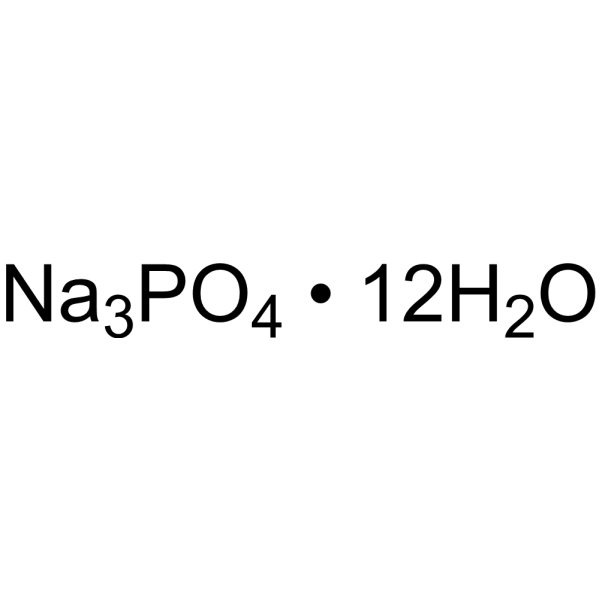 |
Trisodium phosphate dodecahydrate
CAS:10101-89-0 |
|
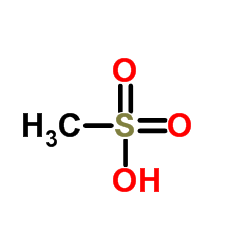 |
Methanesulfonic acid
CAS:75-75-2 |
|
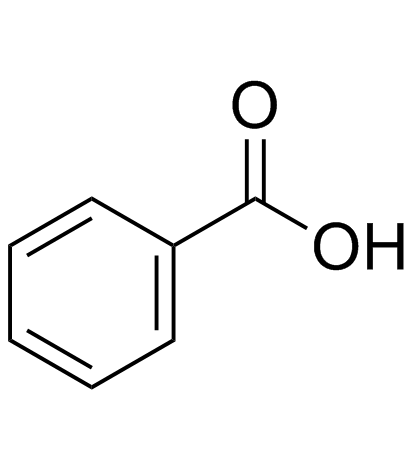 |
benzoic acid
CAS:65-85-0 |
|
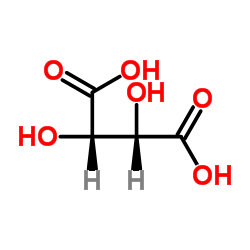 |
Tartaric acid
CAS:87-69-4 |
|
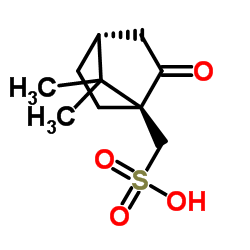 |
D-Camphorsulfonic acid
CAS:3144-16-9 |
|
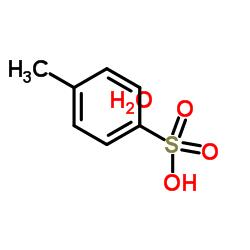 |
p-Toluenesulfonic acid monohydrate
CAS:6192-52-5 |