| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodium chloride
CAS:7647-14-5 |
|
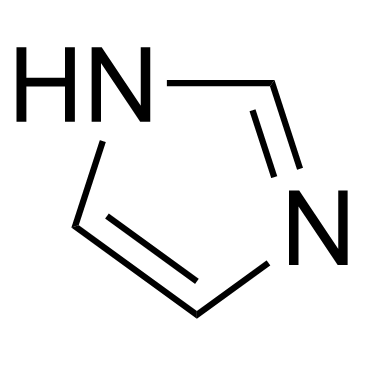 |
Imidazole
CAS:288-32-4 |
|
 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |
|
 |
Sulfur trioxide
CAS:7446-11-9 |
|
 |
o-phospho-l-tyrosine
CAS:21820-51-9 |