Nateglinide--current and future role in the treatment of patients with type 2 diabetes mellitus.
I W Campbell
Index: Int. J. Clin. Pract. 59(10) , 1218-28, (2005)
Full Text: HTML
Abstract
Therapy for type 2 diabetes mellitus should aim to control not only fasting, but also postprandial glucose levels. Nateglinide, a d-phenylalanine derivative, restores postprandial early phase insulin secretion in a transient and glucose-sensitive manner without affecting basal insulin levels. As nateglinide is administered immediately before meals it provides greater lifestyle flexibility than agents that require patients to eat to avoid hypoglycemic events (e.g. long-acting sulfonylureas). In randomised, double-blind trials in patients with type 2 diabetes, nateglinide monotherapy (mealtime treatment of 120 mg three times daily) significantly improved long-term glycaemic control by significantly reducing glyated haemoglobin (HbA 1c) and preventing mealtime glucose spikes. The combination of nateglinide with insulin-sensitising agents, for example, metformin and thiazolidinediones, addresses the dual defects of loss of insulin secretion and insulin resistance to provide optimal management of type 2 diabetes, and more patients achieve HbA 1c goal with nateglinide combination therapy rather than with monotherapy with other oral agents. Nateglinide also restores early insulin secretion and reduces postprandial hyperglycaemia in prediabetic subjects with impaired glucose tolerance (IGT) and appears similarly effective in elderly and non-elderly populations with type 2 diabetes. It has an excellent safety and tolerability profile, with a low propensity to cause hypoglycaemia due to its transient, selective effect on early phase insulin secretion. Nateglinide as monotherapy or combination therapy is an effective option to reduce mealtime glucose in patients with type 2 diabetes. The results of ongoing research into its potential role in delaying progression to overt diabetes, and protecting against cardiovascular events, in prediabetic patients with IGT are awaited.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
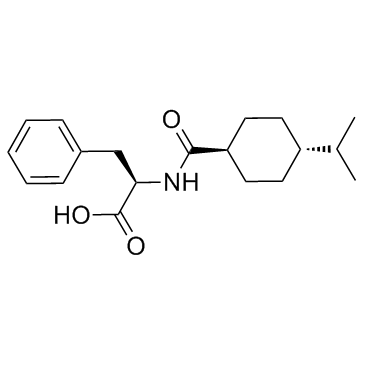 |
Nateglinide
CAS:105816-04-4 |
C19H27NO3 |
|
Formulation and in vitro evaluation of nateglinide microsphe...
2013-11-01 [Pak. J. Pharm. Sci. 26(6) , 1229-35, (2013)] |
|
From evidence assessments to coverage decisions?: the case e...
2012-01-01 [Health Policy 104(1) , 27-31, (2012)] |
|
Efficacy and safety of mitiglinide versus nateglinide in new...
2012-02-01 [Diabetes Obes. Metab. 14(2) , 187-9, (2012)] |
|
Nateglinide (Starlix): update on a new antidiabetic agent.
2003-01-01 [Int. J. Clin. Pract. 57(6) , 535-41, (2003)] |
|
II. Technological approaches to improve the dissolution beha...
2013-09-15 [Int. J. Pharm. 454(1) , 568-72, (2013)] |