| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sucrose
CAS:57-50-1 |
|
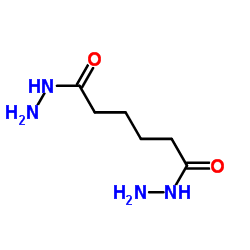 |
Adipohydrazide
CAS:1071-93-8 |
|
 |
dichloroethane
CAS:107-06-2 |
|
 |
MES
CAS:4432-31-9 |