| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Sodium hydroxide
CAS:1310-73-2 |
|
 |
sodium chloride
CAS:7647-14-5 |
|
 |
Dimethyl sulfoxide
CAS:67-68-5 |
|
 |
3-Ethyl-2,4-pentanedione
CAS:1540-34-7 |
|
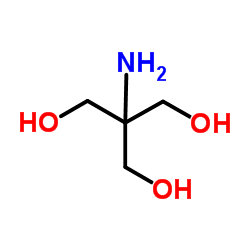 |
Trometamol
CAS:77-86-1 |
|
 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |
|
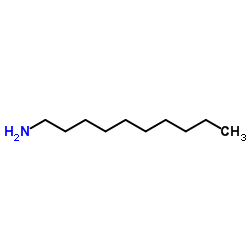 |
decanamine
CAS:2016-57-1 |
|
 |
Ethylenediaminetetraacetic acid
CAS:60-00-4 |
|
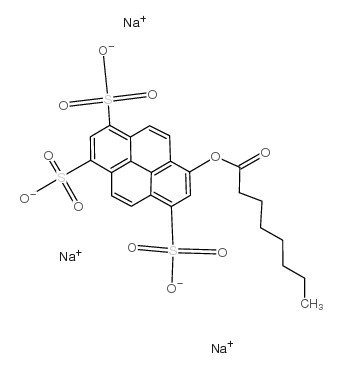 |
8-Octanoyloxypyrene-1,3,6-trisulfonic acid trisodium salt
CAS:115787-84-3 |