| Structure | Name/CAS No. | Articles |
|---|---|---|
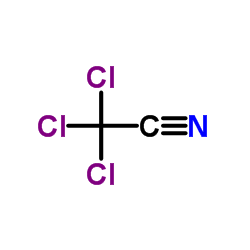 |
Trichloroacetonitrile
CAS:545-06-2 |
|
 |
Acetyl chloride
CAS:75-36-5 |
|
 |
Allyl carbonochloridate
CAS:2937-50-0 |
|
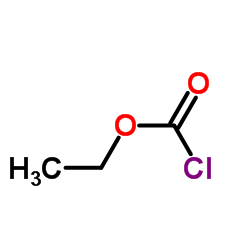 |
Ethyl chloroformate
CAS:541-41-3 |