| Structure | Name/CAS No. | Articles |
|---|---|---|
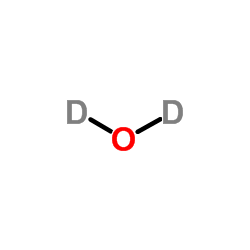 |
Heavy water
CAS:7789-20-0 |
|
 |
Conalbumin, from chicken egg white
CAS:1391-06-6 |
|
 |
Hyaluronidase
CAS:37326-33-3 |
|
 |
hyaluronidase
CAS:37259-53-3 |