| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
L(-)-Tryptophan
CAS:73-22-3 |
|
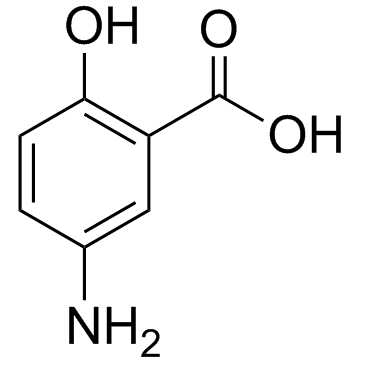 |
5-Aminosalicylic Acid
CAS:89-57-6 |
|
 |
Sulfapyridine
CAS:144-83-2 |
|
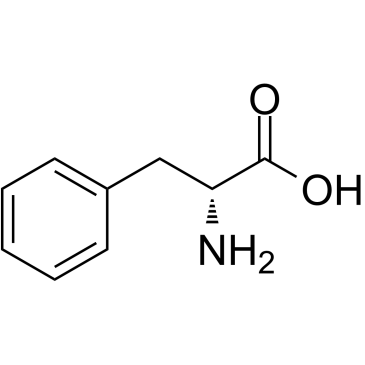 |
D-phenylalanine
CAS:673-06-3 |