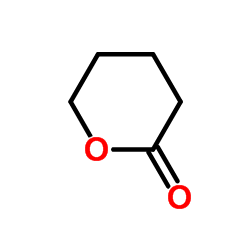
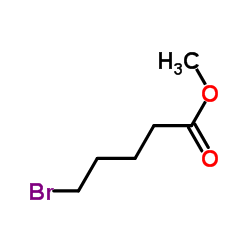
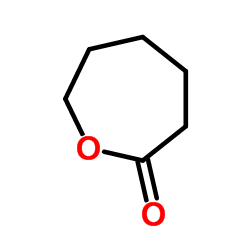
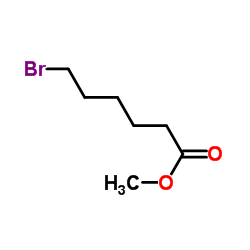
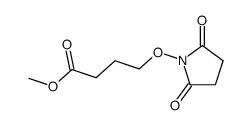
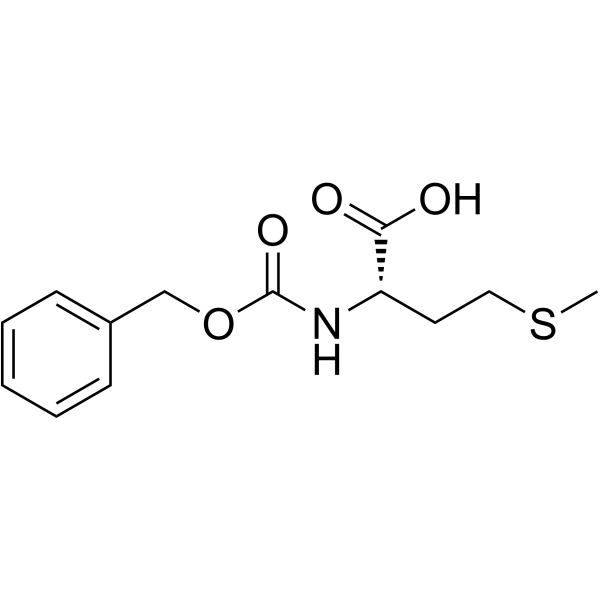
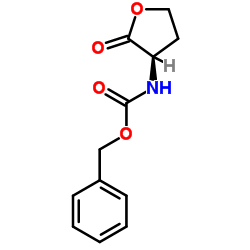
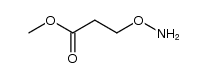
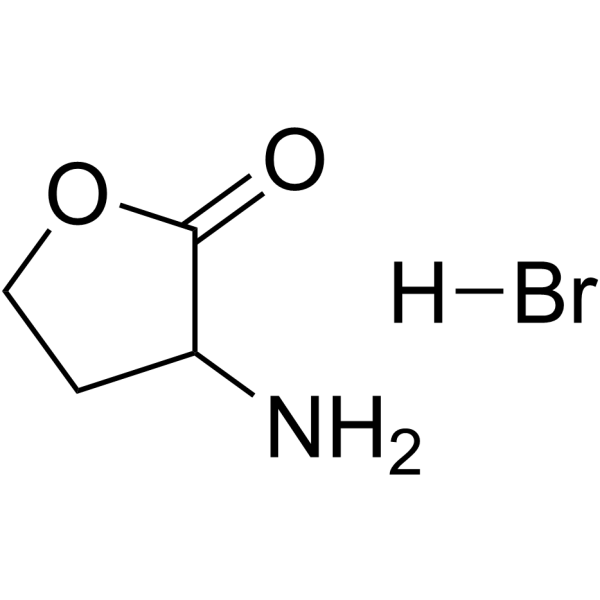|
~56% |
|
~% |
|
~88% |
|
~95% |
|
~97% |
|
~44% |
|
~20% |
|
~45% |

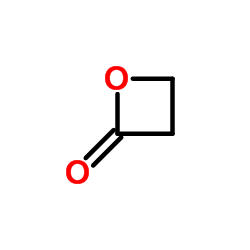
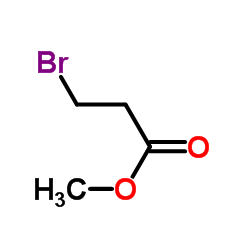

![Methyl N-{[(2-methyl-2-propanyl)oxy]carbonyl}homoserinate Structure](https://image.chemsrc.com/caspic/234/120042-12-8.png)